Long-acting pharmacotherapies targeting incretin hormones like glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP) have revolutionized treatments for obesity and type 2 diabetes (T2D).
These therapies have provided millions with sustained weight loss and better glucose management. For many, GLP-1 receptor agonists offer a lifeline, achieving weight loss comparable to bariatric surgery.
Yet, the success of these drugs falls short for a significant population—those living with both obesity and T2D. Despite affecting over 380 million people globally, this group experiences diminished weight-loss benefits from current therapies.
This disparity highlights an urgent need for novel treatments. One major limitation of current pharmacotherapies is their focus on appetite suppression without addressing energy expenditure.
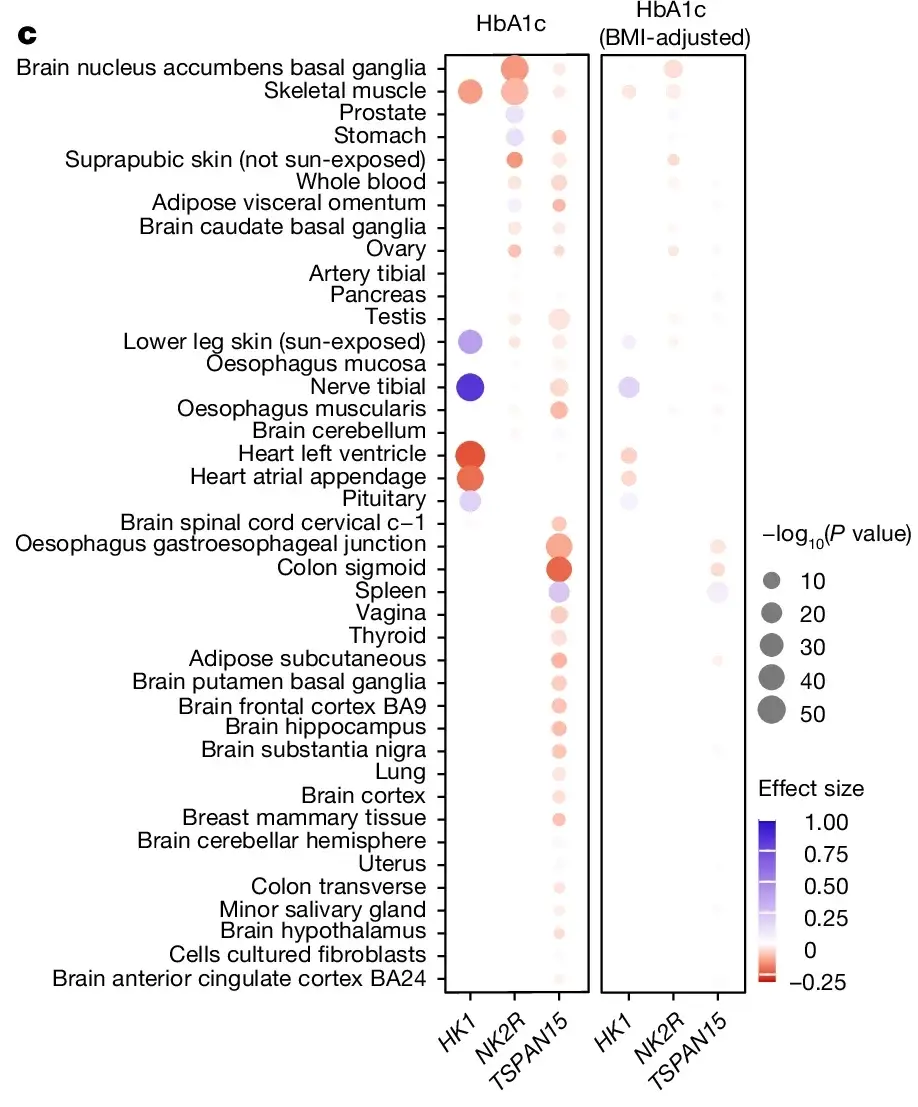
Over the past 40 years, basal metabolic rates have steadily declined, further complicating weight management. Although researchers have explored ways to boost energy expenditure, such as with the mitochondrial uncoupler dinitrophenol or beta-adrenergic activation, these approaches have faced safety and efficacy challenges.
Recent advances in glucagon receptor (GCGR) agonists show promise for enhancing catabolic metabolism, but concerns persist. Potential side effects include increased heart rates, hepatic glucose production, and loss of lean mass.
Researchers have proposed combining GCGR agonists with insulinotropic agents, such as GLP-1 or GIP, to address these limitations. However, the need for a breakthrough remains critical.
A New Pathway: NK2R Activation
Scientists at the University of Copenhagen have made a remarkable discovery that could reshape this landscape. Their study, published in Nature, introduces a drug candidate targeting the Neurokinin 2 Receptor (NK2R).
Unlike current treatments, NK2R activation enhances energy expenditure alongside appetite suppression, without the side effects of nausea or vomiting. This dual mechanism could be a game changer for patients struggling with weight loss and metabolic control.
“Our discovery addresses two critical gaps in current treatments: safely increasing energy expenditure and controlling appetite without nausea,” explains Associate Professor Zach Gerhart-Hines from the NNF Foundation Center for Basic Metabolic Research. He believes these advancements will make treatments more tolerable and accessible to millions.
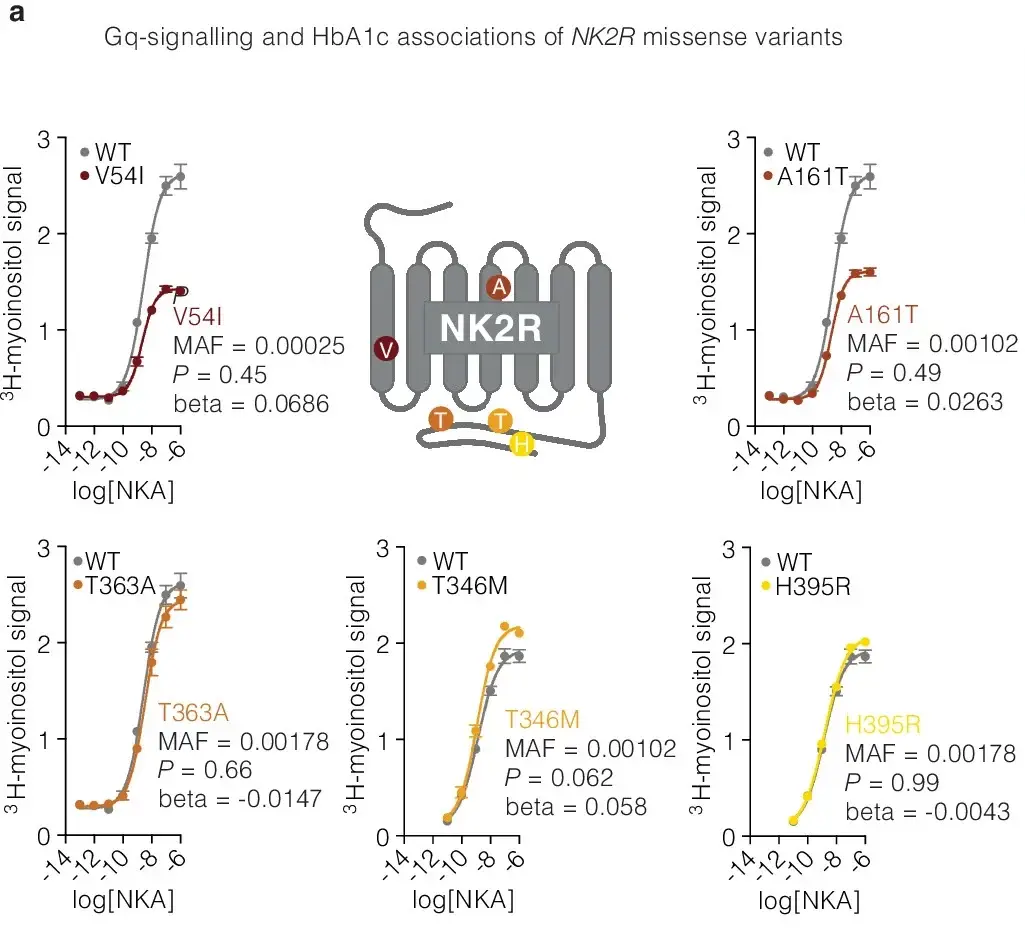
The Energy Balance Equation
Weight management hinges on energy balance: the calories consumed versus those burned. While current incretin-based therapies like Wegovy and Mounjaro effectively reduce calorie intake, boosting calorie burn remains an untapped opportunity. NK2R activation targets this by ramping up calorie expenditure, counteracting the declining metabolic rates seen in recent decades.
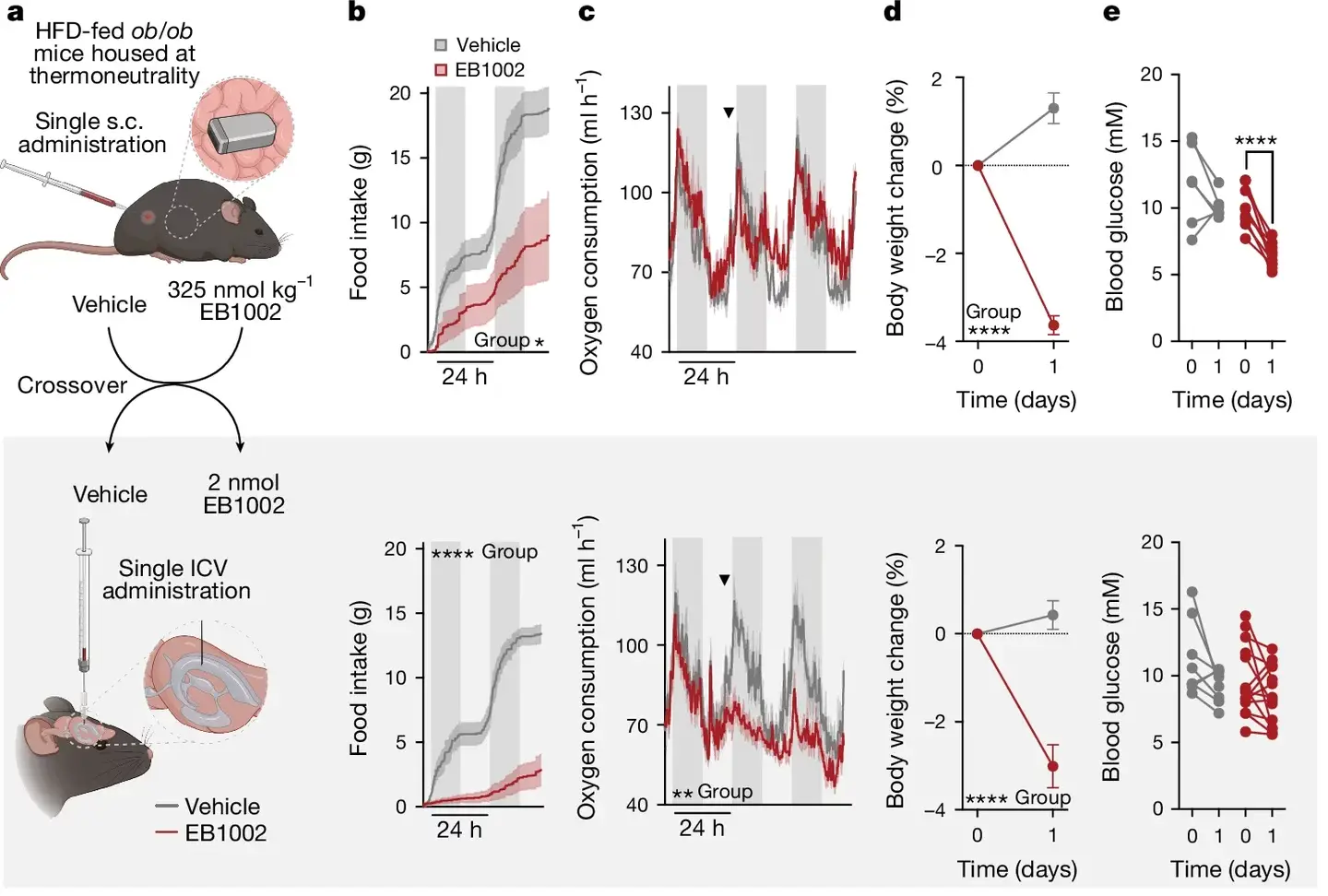
Preclinical trials in mice showed that NK2R activation increased calorie burn and reduced appetite, with no adverse effects. Moving beyond rodent models, the researchers tested the drug on non-human primates with T2D and obesity.
The results were groundbreaking: NK2R activation not only lowered body weight but also reversed diabetes by improving insulin sensitivity and reducing blood sugar, triglycerides, and cholesterol levels.
Bridging the Gap Between Mice and Humans
One of the greatest challenges in drug development is translating findings from animal models to human applications. Frederike Sass, a PhD student and first author of the study, underscores the significance of these results. “The benefits of NK2R agonism translating to diabetic and obese non-human primates is a big step toward clinical trials,” she notes.
This research paves the way for a new generation of cardiometabolic therapies. By targeting NK2R, the University of Copenhagen team has addressed two major hurdles in treating obesity and T2D: enhancing energy expenditure and improving appetite control without compromising patient comfort.
Their work has already led to the creation of several biotech companies, including Embark Biotech, which Novo Nordisk recently acquired to develop next-generation treatments.
Looking Ahead
The implications of this discovery extend far beyond its immediate applications. For nearly 400 million people living with both obesity and T2D, the promise of more effective and tolerable treatments is on the horizon. As researchers continue to explore the potential of NK2R agonists, the landscape of cardiometabolic therapy is poised for a transformative shift.
By addressing both ends of the energy balance equation, this novel approach could help millions achieve sustained weight loss and improved metabolic health.
The University of Copenhagen’s pioneering research not only fills critical gaps in current treatments but also holds the potential to reshape global health outcomes.
source ai